Abrams P. Cardozo L. Fall M. et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Neurourol Urodyn 2010; 21:167-178.
AUGS. Urinary incontinence in women. Practice Bulletin No. 155. American College of Obstetricians and Gynecologists. Obstet Gynecol 2015; 126:e66–81.
Averch D, Stoffel J, Goldman HB, Griebling TL, Lerner L, Newman DK, Peterson AC. Catheter-Associated Urinary Tract Infections: Definitions and Significance in the Urologic Patient. 2014; AUA Guideline.https://www.auanet.org/guidelines/catheter-associated-urinary-tract-infections Accessed October 27 2019.
Ayello EA, Baranoski S, Lyder C, Cuddigan J. Pressure ulcers. In: Baranoski S, Ayello EA, eds. Wound Care Essentials: Practice Principles. Philadelphia, PA: Lippincott Williams & Wilkins; 240-70. 2004.
Barrisford, G. and Steele, G. (2019). UpToDate. [online] Uptodate.com. Available at: https://www.uptodate.com/contents/acute-urinaryretention?search=bladder%20ultrasound&source=search_result&selectedTitle=4~92&usage_type=default[Accessed 18 Oct. 2019].
Black, JM, Gray, M, Bliss, D, et al. MASD part 2: incontinence-associated dermatitis and intertriginous dermatitis: a consensus. J Wound Ostomy Continence Nurs. 2011;38(4),359-370. doi: 10.1097/WON.0b013e31822272d9
Byun SS, Kim HH, Lee E, Paick JS, Kamg W, Oh SJ. Accuracy of bladder volume determinations by ultrasonography: are they accurate over entire bladder volume range? Urology. 2003 Oct;62(4):656-60.
Clark M, Romanelli M, Reger SI, Ranganathan VK, Black J, Dealey C, Microclimate in context. Pressure Ulcer Prevention-A consensus document. Wounds International19-25, 2010.
Coladonato J, Smith A, Watson N, Brown AT, McNichol L, Clegg A, Griffin G, McPhail L, Montgomery TG. Prospective, nonrandomized controlled trials to compare the effect of a silk-like fabric to standard hospital linens on the rate of hospital-acquired pressure ulcers. Ostomy Wound Management. 2012;58(10):14-31.
Colwell, J, Ratliff, C, Goldberg, M, et al. MASD part 3: peristomal moisture– associated dermatitis and periwound moisture–associated dermatitis: a consensus. J Wound Ostomy Continence Nurs. 2011;38(5),541- 553. doi: 10.1097/WON.0b013e31822acd95
Cottenden A. Fader M. Beeckman D. et al. Management using continence products. In: Abrams, P, Cardozo, L, Wagg, A, et al. ed. Incontinence. 6th ed. ICS/ICUD;2017: 2303-2426.
D’Ancona CD, Haylen BT, Oelke M, Herschorn S, Abranches-Monteiro L, Arnold EP, Goldman HB, Hamid R, Homma Y, Marcelissen T, Rademakers K, Schizas A, Singla A, Soto I, Tse V, de Wachter S. An International Continence Society (ICS) Report on the Terminology for Adult Male Lower Urinary Tract and Pelvic Floor Symptoms and Dysfunction. Neurourol Urodyn. 2019 DOI: 10.1002/nau.23897
Diaz, DC Robinson, D. Bosch, R, Costantini, E, Cotterill, N Espuna-Pons, M, Kocjancic, E, Lemos, N, Tarcan, T, Yoshida, M. Initial assessment of urinary incontinence in adult male and female patients. In Abrams, P, Cardozo, L, Wagg, A, Wein A. eds Incontinence 6th ed ICS/ICUD.2017; 497-540.
Dobrowolski ZF; Weglarz W; Jakubik P; Lipczynski W; Dobrowolska B. BJU International 2002; 89, 752-4.
Edsberg, L. E., Black, J. M., Goldberg, M., McNichol, L., Moore, L., & Sieggreen, M. (2016). Revised National Pressure Ulcer Advisory Panel Pressure Injury Staging System: Revised Pressure Injury Staging System. Journal of Wound, Ostomy, and Continence Nursing: official publication of The Wound, Ostomy and Continence Nurses Society, 43(6): 1-13.
*Edsberg LE, Black JM, Goldberg M, McNichol L, Moore L, Sieggreen M. Revised national pressure ulcer advisory panel pressure injury staging system. J Wound Ostomy Continence Nurs 2016;43(6):585-597.
Ferrarin M, Ludwig N. Analysis of thermal properties of wheelchair cushions with thermography. Med Bio Eng Comput 2000;38(1):31-34
Fisher SV, Szymke TE, Apte SY, Kosiak M. Wheelchair cushion effect on skin temperature. Arch Phys med Rehabil 1978; 59(2):68-72
Gerhardt LC, Strassle V, Lenz A, Spencer ND, Derler S. Influence of epidermal hydration on the friction of human skin against textiles. J.R.Soc. Interface (5), 1317-1328, 2008.
Gormley, Em, Lightner, DJ, Burgio, KL, Chai, TC, Clemens, JQ, Culkink DJ, Das, K, Foster, HE, Scarpero, HM, Tessier, CD, Vasvada, SP. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU Guideline. 2014
Gray, M, Black, JM, Baharestani, M, et al. Moisture-associated skin damage overview and pathophysiology. J Wound Ostomy Continence Nurs. 2011;38(3), 233-241. doi: 10.1097/WON.0b013e318215f798
Gray M, Kent D, Ermer-Seltun J, McNichol L. Assessment, selection, use, and evaluation of body-worn absorbent products for adults with Incontinence. J Wound, Ostomy Continence Nurs. 2018;45(3):243-264.
Gray M, Skinner C, Kaler W. External Collection Devices as an Alternative to the Indwelling Urinary Catheter. Journal of Wound, Ostomy and Continence Nursing 2016; 43(3): 301-7.
Hagedorn JC, Wessels H. A contemporary update on Fournier's Gangrene. Nature Reviews in Urology 2017; 14: 205-14.
Haylen BT et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 2010; 29:4–20.
Haylen BT, Frazer MI, MacDonald JH. Assessing the effectiveness of different urinary catheters in emptying the bladder: an application of transvaginal ultrasound. Br J Urol. 1989 Oct;64(4):353-6.
Irion, G. Etiologies of common wounds. In: Comprehensive Wound Management. 2nd ed. Thoroughfare, NJ:Slack; 2010:73-148.
Johnson, E. The Condom Catheter: Urinary Tract Infection and Other Complications. Southern Medical Journal, Vol 76, NO. 5, May, 1983
Kosiak M. Etiology and pathology of ischemic ulcers. Arch Phys Med Rehabil;40:62-9, 1959.
Kredo, T., Berhardsson, S., Machingaidze, S., Young, T., Louw, Q., Ocodo, E., and Grimmer, K. (2016). Guide to clinical practice guidelines: the current state of play. Ing J Qual Health Care; 28(1): 122-128. doi: 10.1093/intqhc/mzv115
Lucas, MG, Bedretdinova, D, Bosch, JIHR, Burkhard,F, Cruz, F, Nambiar, AK deRidder, DJMK, Tubaro, A, Pichard, RS. Guidelines on Urinary Incontinence. European Association of Urology 2013
Maklebust J. Pressure ulcers: etiology and prevention. Nurs Clin North Am;22:359-77, 1987.
McNichol L, Lund C, Rosen T, Gray M. Medical Adhesives and Patient Safety: State of the Science Consensus Statements for the Assessment, Prevention, and Treatment of Adhesive-Related Skin Injuries. J Wound Ostomy Continence Nurs 2013,40(4):365-380. doi: 10.1097/WON.0b013e3182995516
McNichol L, Watts C, Mackey D, Beitz JM, Gray M. Identifying the right surface for the right patient at the right Time. J Wound Ostomy Continence Nurs. 2015;42(1):19-37.
Middleton, J, Ramakrushnan, K, Cameron, I. Management of the Neurogenic Bladder for Adults with Spinal Cord Injuries. Chatswood, NSW Australia: Agency for Clinical Innovation. 2013.
National Pressure Ulcer Advisory Panel, European Pressure Ulcer Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Emily Haesler (Ed.).Cambridge Media: Osborne Park, Western Australia;2014,p.84.
National Pressure Ulcer Advisory Panel, European Pressure Ulcer Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Emily Haesler (Ed.).Cambridge Media: Osborne Park, Western Australia; 2014, p.320.
National Pressure Ulcer Advisory Panel, European Pressure Ulcer Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Emily Haesler (Ed.).Cambridge Media: Osborne Park, Western Australia;2014,p.326.
National Pressure Ulcer Advisory Panel (NPUAP),European Pressure Ulcer Advisory Panel (EPUAP), Pan Pacific Pressure Ulcer Injury Alliance (PPPIA),. (2014). Prevention and Treatment of Pressure Ulcers: Clinical Practic Guideline. Washington, DC: NPUAP.
Prentice, D., Sona, C., Wessman, B., Ablordeppey, E., Isakow, W., Arroyo, C. and Schallom, M. (2017). Discrepancies in measuring bladder volumes with bedside ultrasound and bladder scanning in the intensive care unit: A pilot study. Journal of the Intensive Care Society, 19(2), pp.122-126.
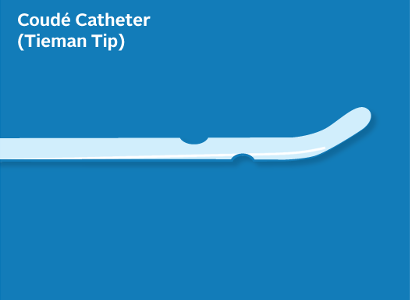
Rew M, Lake H, Moore KB. The use of Tiemann tip catheters for male self-catheterization. British Journal of Nursing. 2018;27(9): S18-S25.
Saint S; Krein SL; Fowler KE; Colozzi J; Ratz D; Lescinskas E; Chrouser K; Trautner BW. Condom Catheters versus Indwelling Urethral Catheters in Men: A Prospective, Observational Study. Journal of Hospital Medicine (Online). 14:E1-E4, 2019 Mar 20.
Schade EB, Oliver-McNeil, S, Benkert T, George N. A quality improvement project to educate nurses on coudé catheter placement. Urologic Nursing. 2015; 35(4): 187-203.
Tailly T. Denstedt JD. Fundamentals of urinary tract drainage. In: Wein A. Kavoussi LR. Partin A. et al. Urology. 11th ed. Philadelphia: Elsevier; 2016: 119-135.e7.
Taylor D, Sierra T, Duenas-Garcia O et al. Accuracy of bladder scanner for the assessment of postvoid residual volumes in women with pelvic organ prolapse. Female Pelvic Med Reconstr Surg. 2018; 25(5):388-391.
The Free Dictionary. Intake and output. Medical Dictionary for the Health Professions and Nursing, Farlex, 2012, https://medical-dictionary.thefreedictionary.com/intake+and+output , Accessed February 2 2020.
Thornburn P, Fader M, Dean G, Brooks R, Cottenden A. Improving the performance of small incontinence pads: a study of “wet comfort”. J Wound Ostomy Continence Nurs 1997 Jul;24(4):219-25
Tse, V, King, J, Dowling, C. English, S. Gray, K, Milard, R, O’Connell, H, Pillay, S, Thavaseelan, J. Conjoint Urological Society of Australia and New Zealand (USANZ) and Urogynaecological Society of Australasia (UGSA) Guidelines on the management of adult non-neurogenic overactive bladder. BJU Int 2016; 117: 34–47
Wagg A. Chen LK. Johnson T. et al. Incontinence in frail older persons. In: Abrams P. Cardozo L. Wagg A. et al. ed. Incontinence. 6th ed. ICS/ICUD; 2017: 1309-1442.
Waites KB, Canupp KC, DeVivo MJ. Epidemiology and risk factors for urinary tract infection following spinal cord injury. Arch Phys Med Rehabil. 1993 Jul;74(7):691-5.
Yates, S., McNichol, L., Heinecke, S. B., & Gray, M. (2017). Embracing the Concept, Defining the Practice, and Changing the Outcome: Setting the Standard for Medical Adhesive-Related Skin Injury Interventions in WOC Nursing Practice. J Wound Ostomy Continence Nurs. official publication of The Wound, Ostomy and Continence Nurses Society, 44(1): 13-17.
Zhong, W., Xing, M.M.Q., Pan, N.& Maiback, H.I., Textiles and Human Skin, Microcliomate, Cutaneous Reactions: An Overview, Cutaneous and Ocular Taxicology, (25), 23-29, 2006.